Results published in 2015 in PNAS
By coupling experimental and numerical approaches, researchers from the Laboratory for Interdisciplinary Physics and their colleagues from Paris have recently elucidated the mechanisms by which a cell adapts its shape and regulates its traction forces to a substrate's stiffness.
Living cells, just like muscles, exert forces on their surroundings. This mobility is at the heart of many biological processes such as cell division, embryogenesis, healing, infection, and immunity.
Although the molecules involved are of the same nature in muscles and non-muscle cells, their organisation is quite different: muscle cells have crystalline solid-like sarcomeres, whereas non-muscle cells have a disordered liquid-like actomyosin network.
Cells and muscles have similar mechanics
A group of researchers from the LIPhy and their colleagues from the MSC have recently shown that in spite of these major differences, the key motor properties of muscles and cells result from similar mechanical phenomena. By comparing experiments and the predictions of a rheological model, they have been able to dissect and quantify the energy usage of a cell when pulling on its substrate, and to explain its amazing versatility and resilience regarding abrupt changes of its near environment.
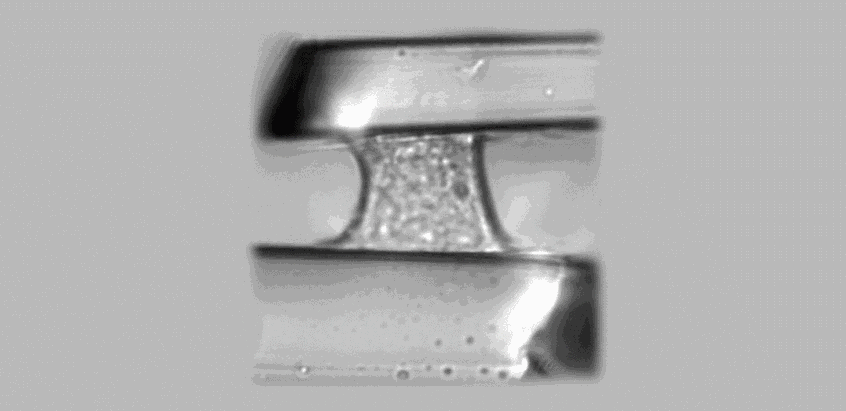
Figure caption
Transmission microscopy image of a single cell adhering between two parallel microplates and submitted to a vertical traction force. The upper microplate is used as a nanonewton force sensor to measure the cell’s response (Credit: MSC Paris Diderot)
Reference
J. Etienne, J. Fouchard, D. Mitrossilis, N. Bufi, P. Durand-Smet and A. Asnacios: Cells as liquid motors. Mechanosensitivity emerges from collective dynamics of actimyosin cortex. PNAS 112(9):2740–2745.



